

AND GASTROINTESTINAL EVENTS
including Boxed WARNING
PROFESSIONALS ONLY
ABDOMINAL/PELVIC
STUDY FINDINGS:
Dyloject can be used without opioids to
deliver substantial
pain relief 1,5
Studied for the treatment of moderate to severe postoperative pain after abdominal or pelvic surgery1
Efficacy
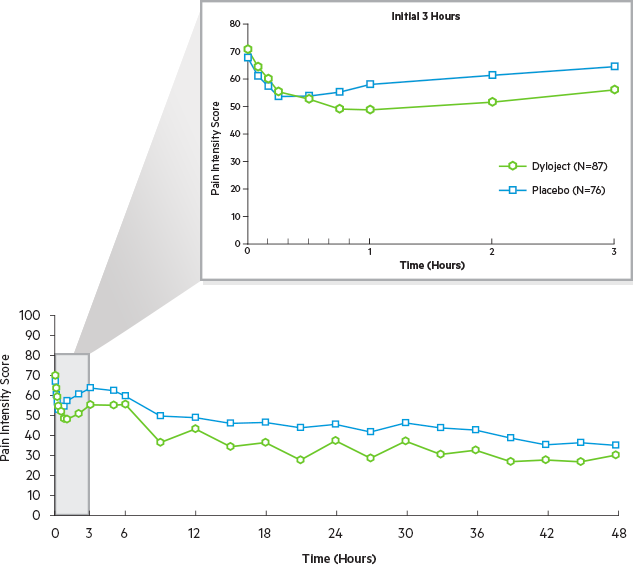
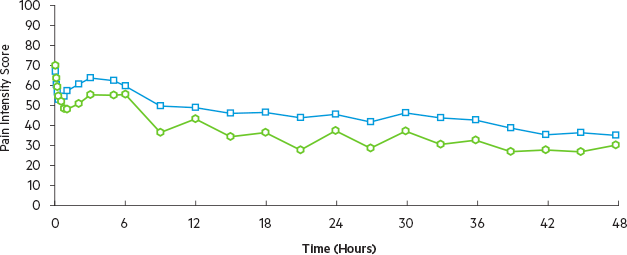
Efficacy was demonstrated by a reduction in pain intensity as measured by the sum of the pain intensity differences over 0 to 48 hours in patients receiving Dyloject as compared to placebo.1
| Surgical Procedures5 | Age range1 | |
|---|---|---|
| Abdominal hysterectomy (25.7%) | Abdominal surgery (8.4%) | 18 to 65 (mean age of 43) |
| Vaginal hysterectomy (17.9%) | Partial colectomy (3.2%) | |
| Myomectomy (14%) | Inguinal hernia repair (13.9%) | |
| Pelvic surgery (6.1%) | Ventral hernia repair (2.0%) | |
| Salpingo-oophorectomy (2.8%) |
Surgical Procedures5
Age range1
The mean baseline pain
intensity on the VAS
was 68 mm (range 50 to 100 mm).1
50 mm≤VAS<70 mm.5
Mean SPID over the first 48 hours significantly greater for Dyloject vs. placebo (P=0.0001)
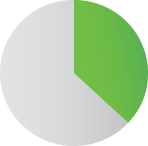
37% of Dyloject patients
did not require opioids1
37%
Dyloject patients not
requiring morphine
63%
Dyloject patients
requiring morphine
dyloject ±
rescue morphine
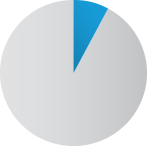
8%
Placebo patients not
requiring morphine
92%
Placebo patients
requiring morphine
PLACEBO ±
rescue morphine
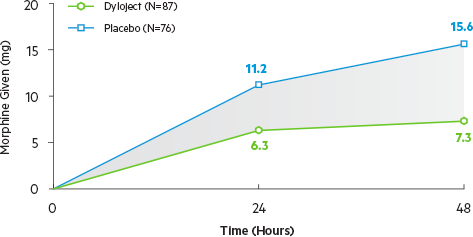
P≤0.0001 vs. placebo for all time intervals
Median time to first rescue dose was 144 minutes for Dyloject vs. 127 minutes for placebo (P=0.057).5
